Radiotherapy is a safe and effective form of treatment for many types of cancer.
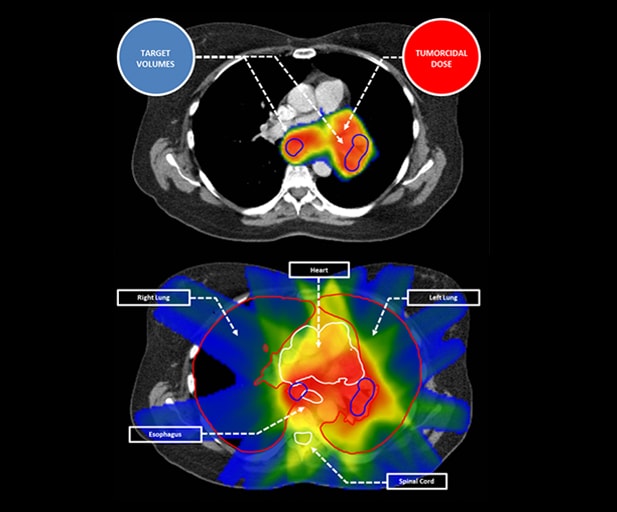
Radiation necessarily hits normal tissues when targeting tumor volumes.
This requires balancing the pursuit of tumorcidal dose to the target with sparing of normal tissues.
Technology adoption, practice patterns, and standards of care differ across geographies and clinics.
The result is large variability in the radiation dose received by study subjects.
This variability has significant implications for both patient response and study endpoints, and is critical to understand and document.